

Paris, October 1st, 2020
The French start-up Cardiawave has developed a highly innovative and non-invasive medical device for the treatment of aortic stenosis using a unique ultrasound technology. Following the success of its first clinical trials in 2019, Cardiawave has secured 8 million euros in funding in 2020.
Cardiawave has developed Valvosoft®, a revolutionary and non-invasive, real-time image-guided medical device using ultrasound technology to treat aortic stenosis, a life-threatening heart disease which affects over 10million people in Western countries. This unique method could be a complementary approach to the current invasive valve replacement (open-heart surgery or transcatheter aortic valve replacement – TAVR). Located in the business incubator of Paris Biotech Santé at the Cochin Hospital in Paris (France), Cardiawave was created in 2014 following research conducted by the Physics for Medicine academic laboratory (INSERM/ESPCI/PSL/CNRS), the Langevin Institute (ESPCI/CNRS) and the George Pompidou European Hospital (HEGP, APHP). Cardiawave has recently expanded its team, its board of directors and its scientific and medical advisory board in order to prepare the next stages of the company’s development which consists in obtaining the CE marking for its Valvosoft® medical device and entering the US market.
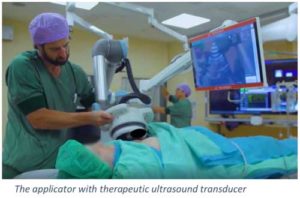
Cardiawave conducted in 2019 the world’s first clinical study of non-invasive treatment in ten elderly patients with severe symptomatic aortic stenosis at the HEGP in Paris (France) and the Amphia Hospital in Breda (The Netherlands). These patients had no therapeutic solution as they were ineligible for valve replacement. The absence of serious adverse events related to Cardiawave’ medical device or to the procedure has proven the safety and the feasibility of the treatment. The
neurological assessment performed in all patients
was satisfactory. With regard to the efficacy, an improvement in the hemodynamic and anatomical parameters was observed in the majority of patients treated, as assessed by an independent certified core-lab. Based on the promising results of this first clinical trial, Cardiawave has been selected to present its preliminary results at prestigious
international cardiology conferences(1).
Cardiawave has obtained funding of 8 million euros in 2020: firstly, thanks to capital increases from its shareholders who have maintained confidence in the company and its management, and secondly, thanks to the grants and subsidies from the European Union, the French National Research Agency (ANR), Bpifrance and a bank loan secured by the French State. This new financing will allow the company to continue the clinical development of its non-invasive therapy for aortic stenosis by improving the first generation of the Valvosoft® medical device and by conducting more clinical trials in Europe and in the United States.
According to Benjamin Bertrand, CEO of Cardiawave: “Our therapy offers tremendous hope for many patients affected by aortic stenosis because, to this day, 16%(2) of them are not eligible for aortic valve replacement. We are very pleased to have secured the necessary funding to maintain our development despite the current COVID-19 pandemic. We have achieved a significant milestone with the success of our first clinical trial. It rewards the great commitment of our teams and partners. Further studies with long-term follow-up and a larger population are required to confirm the safety and performance of our non-invasive therapy. We look confidently ahead to the next steps.”

Located in the business incubator of Paris Biotech Santé in Cochin Hospital and member of the national research consortium RHU Stop-AS, ISO 13485:2016 certified since 2019, Cardiawave has developed a non- invasive medical device for the treatment of valvular heart diseases, especially calcific aortic stenosis, the most prevalent heart valve disease and one of the most common causes of cardiovascular mortality worldwide. Member of the competitiveness cluster MEDICEN Paris Région, Cardiawave employs 26 people and has secured over €22M in funding since its creation at the end of 2014.
This project was supported by the French program “Investissements d’Avenir” as part of the “Concours Mondial de l’Innovation”. It is also supported by the French Government via the National Research Agency (ANR) under the program “Investissements d’Avenir” with the references ANR-16-RHUS-0003_STOP-AS and ANR-17-CE19-0019-03.
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement n° 829492.
contact@cardiawave.com