

The only medical response to severe symptomatic Aortic Stenosis (sSAS) is currently a valve replacement, either by open-heart surgery (surgical aortic valve replacement or SAVR) or by a less-invasive procedure called TAVR (Transcatheter Aortic Valve Replacement).
Unfortunately, not all sSAS patients are eligible for SAVR or TAVR (16%) (1) and some refuse such intervention.
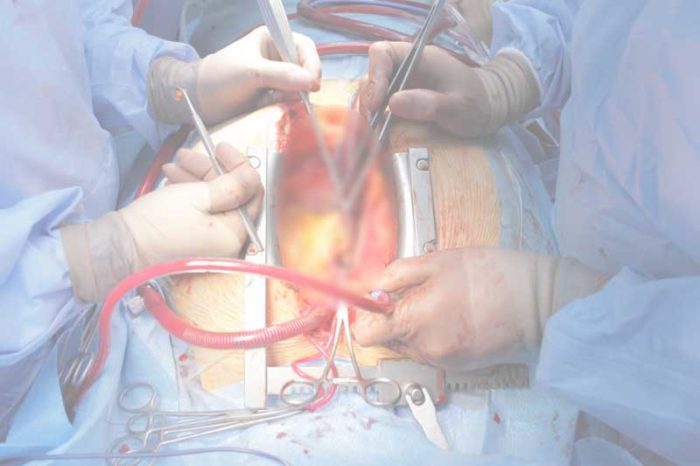
The standard treatment consists in replacing the aortic valve with a biological or mechanical prosthesis during open-heart surgery, performed under cardiopulmonary bypass, which temporarily takes over the function of the heart and lungs while the heart is deliberately stopped to allow for a safe and precise valve replacement (1).
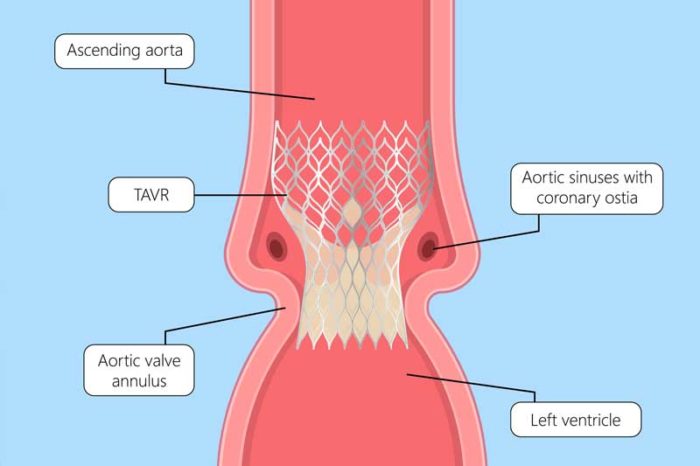
Since 2007, a new technique for minimally invasive endovascular intervention called TAVR has been used with good results. This procedure involves replacing the aortic valve via a percutaneous approach, most commonly through the femoral artery (transfemoral route), which provides access to the aortic valve via the arterial system. Other approaches: Transapical (left ventricular apex), Transaortic (via upper mini-sternotomy or right anterior thoracotomy), Transaxillary or subclavian artery, Transcarotid.
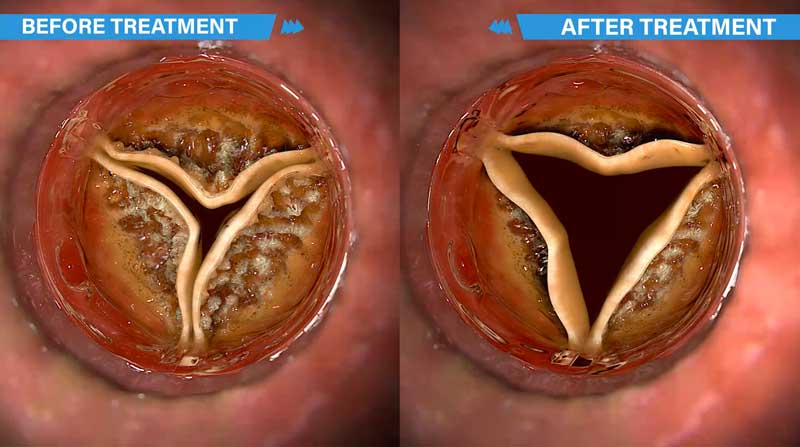
To treat calcific AS, Cardiawave has developed Valvosoft®, a Non-Invasive Ultrasound Therapy (NIUT). NIUT consists in softening the aortic valve, not replacing it. No surgery is required.
Our unique approach could represent both an alternative for patients not recommended or refusing such intervention for SAVR/TAVR (Surgical/Transcatheter Aortic Valve Replacement) and a complementary approach to current procedures to prepare overly calcified aortic valves before the deployment of a TAVR.
Valvosoft® is CE-marked in the European Union when intended for the treatment of patients with severe symptomatic calcific Aortic Stenosis (AS) by non-invasive ultrasound therapy (NIUT). In the United States of America, Valvosoft® is not yet approved by the FDA and is not commercially available.